ORANGE BIOMED UNVEILS WORLD’S FIRST MICROFLUIDIC-BASED A1C TEST AT THE DIABETES TECHNOLOGY MEETING’S STARTUP SHOWCASE
Attendees get an early preview of Orange Biomed’s revolutionary A1C testing method, recognized as a groundbreaking solution with anticipated release in 2025
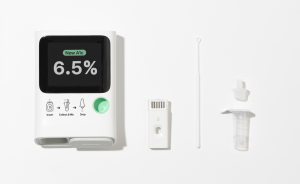
SEATTLE, WA, UNITED STATES, September 10, 2024 /EINPresswire.com/ -- Orange Biomed, inventor of the world's first pocket-sized, single-cell, micro-electro-mechanical A1C analysis device, agnostic to hemoglobin variants — was selected to present groundbreaking research during the Startup Showcase at the 2024 Diabetes Technology Meeting. Attendees will have the in-person opportunity to experience a hands-on demonstration of OBM rapid A1c — a portable, lab-accurate A1C meter featuring breakthrough technology with the potential to transform diabetes care by providing faster, more reliable results. OBM rapid A1c expects to receive market clearance in 2025 and is providing an early preview to researchers and clinicians. Attendees are invited to visit Orange Biomed at the Diabetes Technology Meeting in Burlingame, California, from October 15-17, 2024.“Advancing microfluidic diagnostic technology, we developed the world's first portable and reusable A1C testing system, agnostic to hemoglobin variants. It is as accurate as lab equipment and as convenient as a typical glucometer,” said Yeaseul Park, CEO of Orange Biomed.
OBM rapid A1c leverages advanced microfluidics to perform single-cell analysis of red blood cells, delivering rapid, lab-accurate A1C results from one drop of blood. This technology improves upon existing portable A1C meters, offering enhanced accuracy in point-of-care settings for providers, as well as reliable results for patients who may find it challenging to obtain accurate readings from traditional at-home tests.
Park continued, “Portable A1C tests on the market can generate inaccurate results when patients have disproportionately more hemoglobin variants. Black, Hispanic, and Asian Americans report high rates of hemoglobin variants, increasing the potential of receiving inaccurate A1c readings. Our method seeks to provide accurate readings for everyone – overcoming current gaps in care.”
The Diabetes Technology Meeting connects technology developers and users to facilitate the creation of new and cost-effective tools to help people with diabetes. OBM rapid A1c was selected for the Startup Showcase after being reviewed by the Diabetes Technology Meeting Planning Committee. During the Startup Showcase and booth presentation, Orange Biomed will highlight the technical aspects of OBM rapid A1c and its potential to advance diabetes management. The company’s abstract presentation will offer a detailed overview of the microfluidic components, discussing their capabilities in improving traditional test methods.
Dr. Eunyoung Park, Engineering Team Director at Orange Biomed, will lead technical presentations and discussions on the benefits of microfluidics in A1C testing. “Traditional POC A1C tests also require exact sample sizes to generate accurate results. Patients can struggle to extract the precise amount of blood necessary. Microfluidics enable highly precise, cellular-level analysis, improving the accuracy of results even with inconsistent sample sizes of one drop of blood,” said Park. “This easier approach could reshape diabetes management and diagnostics in the long term, making effective testing more accessible in everyday healthcare settings and for patients on the go.”
About Orange Biomed:
With U.S. headquarters in Seattle, WA, Orange Biomed was launched in 2021 by Duke University alumnus Dr. Unghyeon Ko and Yeaseul Park to solve unmet diabetes-focused healthcare needs. The healthcare startup innovates cutting-edge technology for diabetes management.
In 2024, Orange Biomed acquired an ISO 9001 certification, which certified its quality control capabilities as meeting international standards.
Global studies of OBM rapid A1c have closed with Asan Medical Center and are currently underway in the U.S. market. U.S. FDA clearances of OBM rapid A1c for OTC and Professional usage are anticipated for 2025. Learn more: https://www.orangebiomed.com
About the Diabetes Technology Meeting:
Hosted by the Diabetes Technology Society, a nonprofit organization focused on promoting the development and use of technology in the fight against diabetes. Learn more: https://www.diabetestechnology.org/dtm/welcome-statement.shtml
Shannon Lindahl
Orange Biomed
+1 781-733-6973
shannon@orangebiomed.com
Distribution channels: Business & Economy, Consumer Goods, Electronics Industry, Healthcare & Pharmaceuticals Industry, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release